BOND ANGLES
ABSTRACT:
WHY ARE THERE ALIENS IN CHEMISTRY?!
...Was my immediate message to two A-level students I met at the university open day.
I had just reached the bond angle and molecule shape topic in chemsitry 1 - molecules have some extraterrestrial vibes.
It's a fascinating concept. The geometry of molecules is one I should gel with as an artist.
The angles aren't just random and the atoms aren't just stuck together like you see in ball and stick models.
They're measurable, predictable and repulsive.
METHODOLOGY:
- Watched 2 Youtube videos on A-level molecule shapes and valence shell electron pair repulsion.
Seeing the 3D models and diagrams really helped make sense of how molecules are actually bonded in chemistry.
DISCUSSION AND RESULTS:
How can bond angles actually be measured?
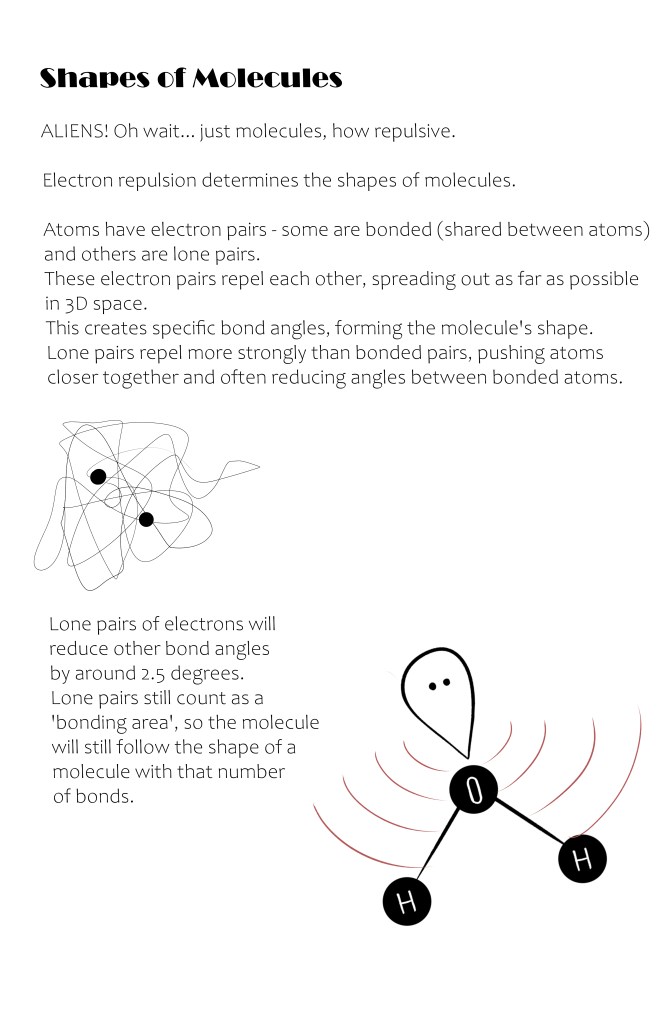
The electrons operate in a cloud of "approximate locations". They aren't static. These fields are then geometircally repelled, creating specific (and accepted) bond angles between each of these locations!?!
This is one of the concepts in chemsitry that make we wonder if it was all made up using conveniently fitting ideas.
Nevertheless, I can see how electron repulsion will push each atoms that are bonded into opposite directions where the force is least. I will also accept that any electron pairs that are not bonded have a stronger repulsion and will narrow these bond angles.
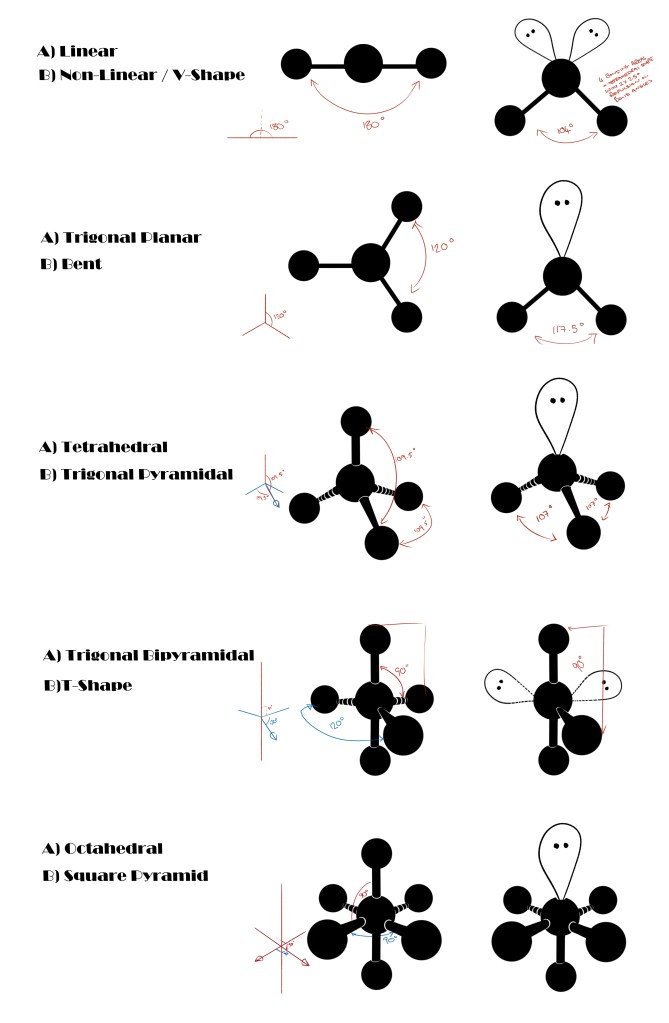
The shapes are based on the number of bonding locations (bonding locations include covalent bonds between atoms and lone pairs of electrons) on a central atom.
For the most part, the shapes these forces create makes sense:
Shape:
Linear
Trigonal planar
Tetrahedral
Trigonal pyramidal
Bent/Angular
Bond Angle(s):
180°
120°
109.5°
107°
104.5°
Example:
Carbon dioxide
Boron TriFl
Methane
Amonia
Water
Bond Angle with Lone Pair:
117.5°
107°
104.5°
Water was the most interesting molecule to learn about.
We've probably all seen a picture of a water molecule - a central oxygen atom and two hydrogen atoms bent below its middle.
But if there are only three atoms, why is it bent? Why not linear?
Although three atoms would typically have two bond locations (between the central atom and the other two atoms), water has four bond locations.
Oxygen is bonded to both hydrogen atoms, but oxygen has 6 electrons in its outer shell. Each hydrogen atom forms a covalent bond with 1 oxygen electron, leaving 4 unbonded electrons around oxygen to pair up.
This means that oxygen has 2 lone pairs and 2 covalent bonds, making 4 bond locations.
But if there are four bonding locations, why is it bent? Why not tetrahedral shape?
Because of the lone pairs! Lone pairs have a greater repulsion that reduces bonded angles by around 2.5° per lone pair.
As oxygen in water has 2 lone pairs, the bond angle between the hydrogens is reduced by 5°.
The tetrahedral shape's bond angle of 109.5° is reduced to 104.5°, creating a bent shape instead.
HOW DOES THIS RELATE TO PHARMACY?! Don't tell me I have to know this.
Drug-receptors.
A lot of medicines work by interacting with receptors in the body using a 'lock and key' method.
This means that the medicines work most effectively when the shape of the molecule fits the shape of the receptor to trigger a response.
Bugger. I am going to have to know this.
LOGICAL CONCLUSIONS:
Bond angles look alien on paper, but they represent an important part of chemistry that will relate to pharmacy.
It's incredible that we can predict and explain molecular shapes based on something as intangible as electron clouds.
However, understanding this has showed me how small changes in geometry can have big effects on function when it comes to a medicine.