HOLY MOLEY!
ABSTRACT:
The mole.
This is a cool unit with an unbelievably large number in chemistry, conceptualised by Avogadro in 1811.
Used to count particles such as: atoms, molecules, ions and formulae, that number is:
602,000,000,000,000,000,000,000 particles.
Just a wee number.
It's a constant - 1 mole of a substances has the same number of particles as 1 mole of another substance.
This makes it useful in: calculating masses, balancing equations and determining formulas.
BUT CAN IT PREVENT A DISPENSING ERROR?
METHODOLOGY:
- Watched 2 Youtube videos on A-level moles.
- Read BBC Bitesize AQA information on moles.
- Completed associated quiz and questions.
- Asked ChatGPT to generate questions to complete, comparing answers after.
- Listened to a 2nd year student warn me that chemistry was HEAVY in first and second year at uni. She said to focus on moles and organic chemistry.
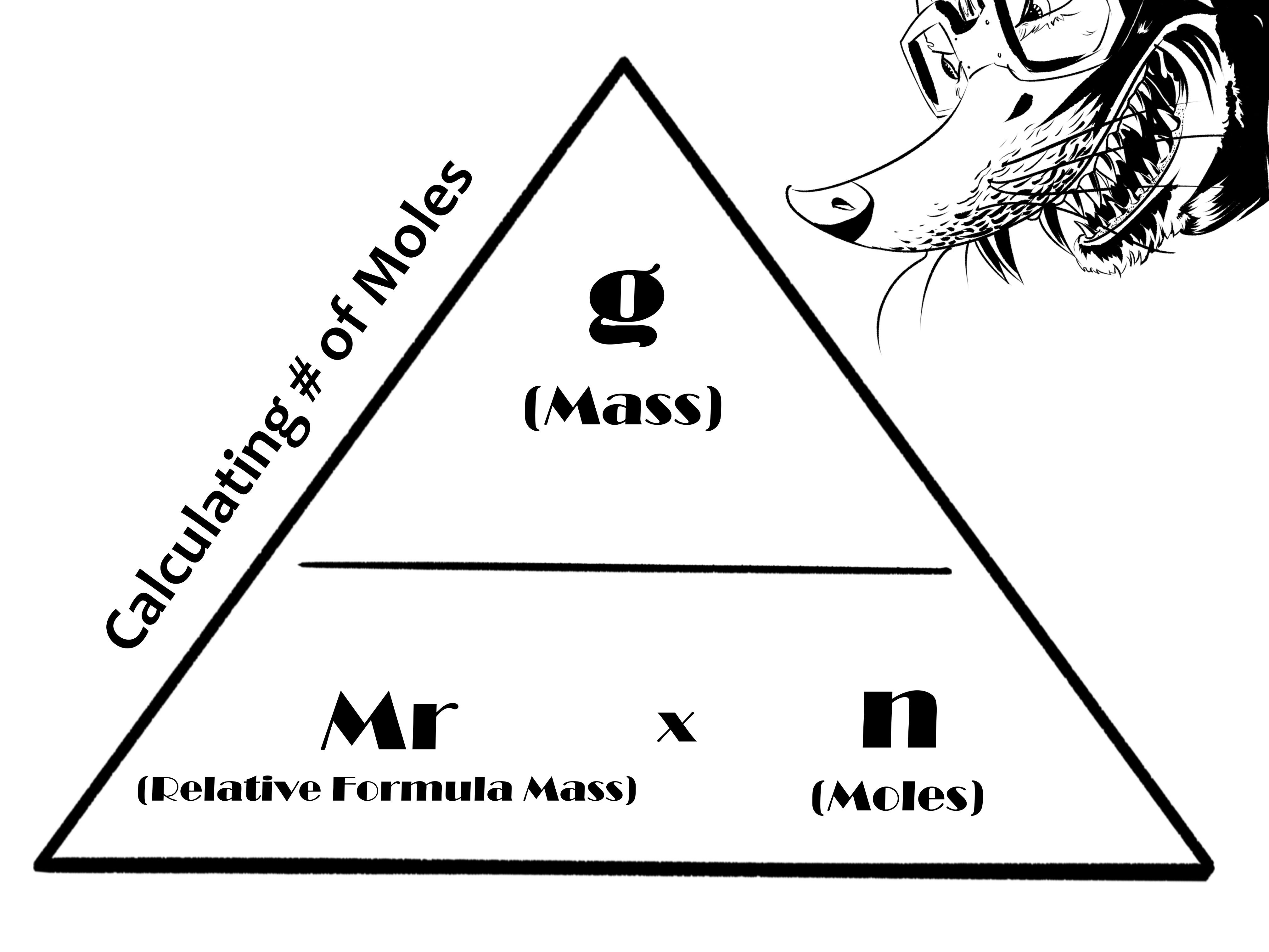
- 'Malicious moles' created to help me remember the mole equations as a 'danger' triangle.
DISCUSSION AND RESULTS:
Thought - If moles are a constant then it can likely be applied to anything. So why don't we use it more to exaggerate in life?
"FML there's 1 mole of plates to wash", "God these shoes stink, it must've had 1 mole of toes inside."
CAN MOLES PREVENT A DISPENSING ERROR?
One item dispensers hate to pick in pharmacy is calcium carbonate and vitamin D preparations.
It's too easy to pick the wrong one.
Not only do they come in multiple forms: caplets, chewable tablets, solutions and effervescsent tablets;
They come in multiple strengths: 1.5g / 400iu, 1.25g / 400iu, 1.25g / 800iu.
BUT THAT'S NOT ALL. They all look the same.
AND THAT'S NOT ALL. Some brands won't show you the strength of calcium carbonate inside. What? Instead they state the equivelant strength of elemental calcium, the element not the compound.
Why do they do this? Is it because they're a supplement or because they're evil?
The prescription states that I need to dispense calcium carbonate 1.25g but the product I hold in my hand has 500mg of elemental calcium.
Is this right? This just asks for errors!
This was the perfect opportunity to test moles!
The mole could confirm if the 500mg elemental calcium I picked up really was equivelant to 1.25g of calcium carbonate.
1.
Work out the relative formula mass (Mr) of calcium carbonate (CaCO3):
Calcium x1 = 40
Carbon x1 = 12
Oxygen x3 = 48
Total Mr = 100
2.
Work out the number of moles of calcium carbonate:
Mass (1.25g) / Mr (100) = 0.0125 moles
3.
Work out the relative formula mass (Mr) of calcium (Ca):
Caclium x1 = 40
Total Mr = 40
4.
As 0.0125 moles of calcium created 0.0125 moles of calcium carbonate, we can work out the mass of calcium:
40 x 0.0125 = 0.5g (500mg) of elemental calcium.
Holy s**t! The formula works.
No dispensing error today! We picked the right item.
LOGICAL CONCLUSIONS:
I understand the concept and how to work out the calculations depending on which information is available.
I've even made it work in practice! But I wouldn't recommend trying to use mole calculations in the middle of busy day at a community pharmacy unless you want to aggrevate the pharmacist.
Despite these successes however, I might be cooked when it comes to knowing when to apply mole calculations in exam-style questions and not just my general curiosity.
One video I watched said "if in doubt, find the moles". This might be one of the most important statements to remember going forward.