ISOTOPES
ABSTRACT:
The atomic twins, isotopes.
Nearly identical in appearance and personality, they differ in their mass.
They have the same number of protons (so they're the same element) but a different number of neutrons, giving them slightly different masses, and in some cases, different properties too.
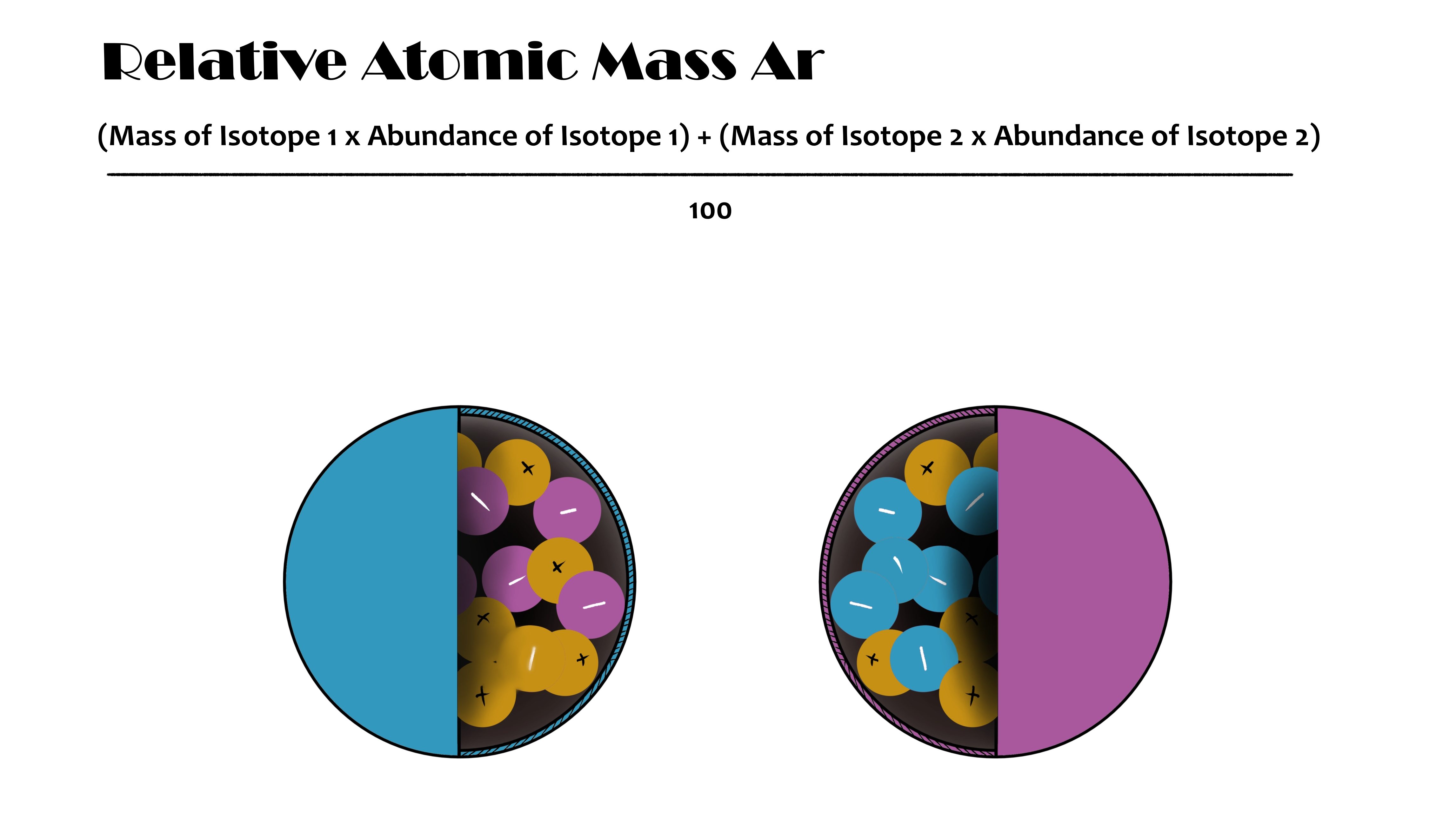
The relative atomic mass that we see on the periodic table is an 'average' mass of all the element's isotopes based on how popular an isotope is in nature.
METHODOLOGY:
- Watched a Youtube video on A-level chemistry.
- Read BBC Bitesize AQA information on isotopes.
- Completed associated quiz and questions on working out relative atomic mass.
DISCUSSION AND RESULTS:
The calculation used with isotopes shows us the relative atomic mass of an element. This is the relative atomic mass we see on the periodic table and is an 'average' mass of the isotopes.
The calculation is 'weighted' towards the most abundant (most nautrally occuring) isotopes so that the relative atomic mass reflects the mass of the most popular versions of an element.
At first I thought isotopes were going to have a groundbreaking difference in behaviour, but it was surprising that chemically they are very alike.
As chemical reactions depend on electron structure, isotopes have the same chemical properties and they usually react in the same way.
It took a bit of extra digging and straying away from pharmacy, but the difference in neutrons does matter in application.
For example, different isotopes of carbon can be used in carbon-dating artifacts. As one of the isotopes are less stable, they tend to decay over time and like the rings of a tree, this can be used to date artifacts.
Radioisotopes (the unstable isotopes) are used in diagnosis and treatments. For example, a hospital I worked in used technetium-99m in their imaging department to show blood flow through organs and identify potential cancers or problems.
LOGICAL CONCLUSIONS:
I understand that isotopes are 'different versions' of the same element but with different masses due to extra or less neutrons.
I know how to work out the relative atomic mass for an element by calculating an 'average' mass of their isotopes, but I don't yet see how this calculation will come into play in pharmacy specifically.